Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Nanometal Decorated Carbon Nanomaterials Generated from Agro-Waste as Excellent Adsorbent for Toxic Metal Ions
Authors: Bholanath T. Mukherjee, Arati D. Nandapure, Pooja O. Jayswal
DOI Link: https://doi.org/10.22214/ijraset.2023.56385
Certificate: View Certificate
Abstract
The effluent water contains heavy metals, dyes and other toxic chemicals which are harmful for humans and environments. By using adsorption technique, one can easily remove most of the toxic materials including metal ions. Adsorption depend upon the physical interaction between adsorbate and adsorbent. Due to specific structural properties, Carbon nanomaterials are used as the adsorbents for removal of metal ions present in effluents as well as for purification of water. In the present work, Pb2+ and Cr6+ ions were removed from aqueous medium using nanomaterials which is a nanocomposite of Carbon nanomaterials decorated with nanometal (Cu). The Carbon nanomaterials were prepared from agro-wastes sugarcane bagasse. It can remove metal ions in the range of 85 to 90 % from solution having low concentrations of 12-14 ppm. Adsorption of metal ions were found to depend on the pH of the aqueous media. It can show maximum adsorption at pH 6 for Pb2+and at pH 1-3 for Cr6+. Morphological features of nanoparticles were analyzed using SEM, HRTEM, Raman, XRD and Specific surface area was determined using BET.
Introduction
I. INTRODUCTION
There are many metals utilized in industries which are toxic and non–biodegradable. For manufacturing of paints and batteries and in many other industries such toxic metals are extensively used. These metals are harmful to animals and human beings. These remain in the environment for a number of years. Such metals are highly toxic and may cause damages to the central nervous system, kidneys, lungs, and other organs.[Reglero M.M et al. 2009; Gybina A.A and Prohaska J.R 2008; Kampa M and. Castanas E 2008; Afroze S and Sen T.K 2018] Many of these metal ions are carcinogenic in nature.[Cocarta D et al. 2016]
Pb2+causes anemia, hypertension, renal impairment, immune-deficiency and toxicity to the reproductive organs. The neurological and behavioral effects of lead are believed to be irreversible.[M Samuel Collin et al. 2022]
Cr6+ is responsible for respiratory and pituitary cancer, eye, kidney and liver damage. Respiratory, nose and skin irritation. It also causes upper abdominal pain.[Sinicropi M.S et al. 2010; Vendruscolo F et al. 2017]
These waste waters or effluents containing heavy metal ions are discharged into natural water bodies, many a times in an untreated form. The concentrations of these metal ions may get enriched by precipitation, adsorption and become life threatening to the aquatic organisms. These may then enter the food chain and cause severe health issues. Thus, the removal of such toxic metal ions from wastewater before discharging in water bodies is becoming an outmost necessity.
There are many techniques for removal of such toxic metals viz. reverse osmosis, reduction, ion-exchange, precipitation and membrane filtration. But the main disadvantage of these techniques is operational and maintenance cost.[Jadhav SV et al. 2015; Weng C-H et al. 2008]. Adsorption is one of the easiest techniques for removal of such toxic metals. The use of Nanotechnology is one of the best solutions for removal of such toxic metal by adsorption method. [Kunduru KR et al.-2017; Dasgupta N et al. 2017; Qu X et al. 2013; Zhang Y et al. 2016 ]
In the present work, preparation of Carbon nanomaterials (CNMs) was carried out by using sugarcane bagasse which is an agro- waste and then the CNMs were decorated with copper nanometals which increases the adsorption capacity of toxic metals, such as Pb2+, Cr6+. The large specific surface area of the material was confirmed by BET, further substantiating it as an excellent candidate for adsorption.
II. EXPERIMENTAL TECHNIQUES
All the chemicals used were of AR grade.
A. Synthesis of Carbon nanomaterials
For removal of toxic metals, CNMs were used. There are many methods for synthesis of CNMs from agro waste.[Nady A. Fathy et al. 2020]
In the present work, the CNMs were prepared from agro-waste, sugarcane bagasse. Bagasse was collected from local juice center. Bagasse was heated in an inert atmosphere at 7300C in a furnace and then treated by using alkali solution. This carbon was impregnated with copper and annealed in presence of CO2 at 7300C. Metal decorated CNM is highly porous in nature.
B. Removal of toxic metals
1000 ppm stock solutions of Pb(NO3)2 and of K2Cr2O7 were prepared initially. Then, solution in the range of 10-14 ppm of metal ions were prepared from the stock solution.
The experiments were carried out by using 0.1 to 0.2 g of CNMs decorated with copper nanometals. 10 cm3 of solutions were used for estimation. The solutions were shaken using electronic mechanical shaker for 30 min. pH of the solution ranges from 1-6 pH and the pH was adjusted by using hexamine powder in case of Pb2+ and concentrated sulfuric acid for Cr6+. The solution was filtered and the filtrate was analyzed for adsorption using an Inductively Coupled Plasma Atomic Emission Spectrophotometer and UV Visible Double Beam Spectrophotometer.
The adsorption was calculated by initial and final concentration of metal ions.[Jie Ma et al 2019; P. M. Shukla and S.R Shukla- 2013]
% Adsorption = [Ci -Ce] / Ci × 100
Ci and Ce is initial and final concentration of metal ions respectively.
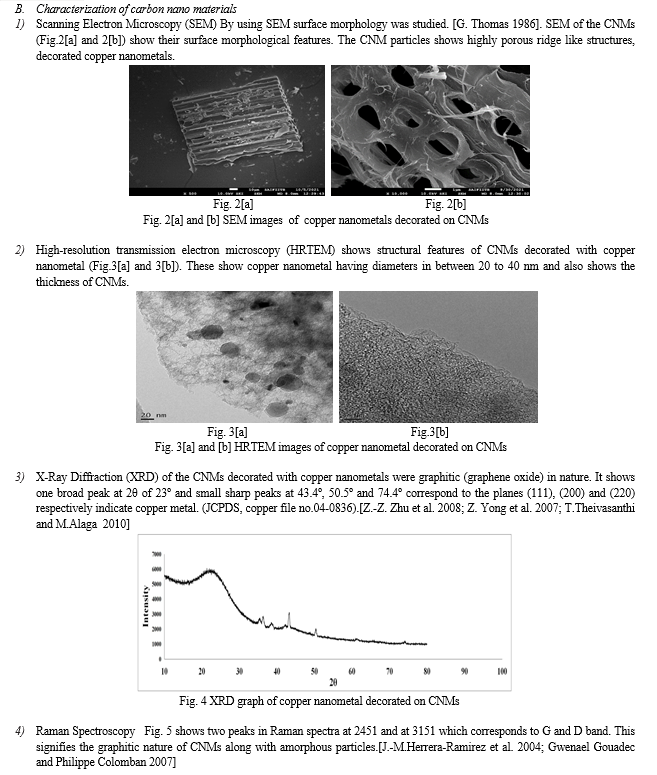
III. RESULT AND DISCUSSION
Activation of carbon and then decoration of metals on CNMs increases the active surface area on CNMs,[K.R. Jagdeo et al.2021] which increases % of adsorption of toxic metals.
Depending on the effect of pH and contact time it was found that CNMs decorated with copper nanometals shows very high adsorption for Pb2+and Cr6+. Percentage of adsorption for Pb2+ and Cr6+ were found to be 90% and 85 % respectively.
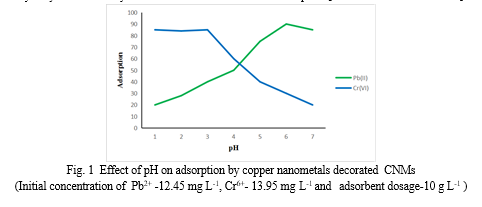
A. Effect of pH on adsorption
pH of solution plays an important role in adsorption process by affecting active site of CNMs.[Ding C et al. 2016; Palin D et al. 2016].From fig. 1 it was observed that for Pb2+ions the percentage of adsorption increases as pH increases. This is possibly due to presence of low Proton density in the solution, more active site was available on CNMs for metal ions adsorption. Due to higher Proton density in the solution at lower pH, there was competition between Proton and metal ions for active site of CNMs.
In case of Cr6+ions, at a lower pH (1-3) value, Cr6+ exists mainly as negatively charged HCrO4- ions and CrO72- ions. Since, the surface of adsorbent is charged positively, the percentage of adsorption was high at lower pH. At basic pH condition, the suppression of the hydrolysis of Cr6+ may be the reason for the decreased adsorption.[L Anah and N Astrini 2017]

IV. ACKNOWLEDGEMENT
The authors sincerely thank SAIF, IITB for carrying out the characterization of the synthesized CNMs.
Conclusion
In present work, sugarcane bagasse (an agro-waste) was used as precursor for synthesis of CNMs, a very cost-effective material. The CNMs decorated with copper nanometal was found to be a good adsorbent for toxic metal ions. Due to the high specific surface area and severely porous surface, it was able to remove the toxic ions in the range of 85 to 90% but is pH dependent. Percentage of adsorption for Pb2+ and Cr6+ were found to be 90% and 85 % respectively.
References
[1] Afroze S., Sen T.K.“ A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents”. Water Air Soil Pollut.;vol.229;issue 7;pp.225. doi: 10.1007/s11270-018-3869-z. 2018 [2] Cocâr?? D., Neam?u S., Deac A.R.,“ Carcinogenic risk evaluation for human health risk assessment from soils contaminated with heavy metals.” Int. J. Environ. Sci. Technol.;vol.13;pp.2025–2036. doi: 10.1007/s13762-016- 1031-2, 2016. [3] Dasgupta N, Ranjan S, Ramalingam C ,“ Applications of nanotechnology in agriculture and water quality management”. Environ Chem Lett ;vol.15 issue 4;pp.591–605,2017. [4] Ding, C.; Cheng, W.; Wang, X.; Wu, Z. Y.; Sun, Y.; Chen, C.; Wang, X.; Yu, S. H.“ Competitive sorption of Pb(II), Cu(II) and Ni(II) on carbonaceous nanofibers: A spectroscopic and modeling approach”. J. Hazard. Mater ,vol.313;pp.253–261;doi: 10.1016/j.jhazmat.2016.04.002 ;2016. [5] Eleni A. Deliyanni , George Z. Kyzas , Kostas S. Triantafyllidis and Kostas A. Matis ,“Activated carbons for the removal of heavy metal ions: A systematic review of recent literature focused on lead and arsenic ions”, Open Chemistry, vol-13 issue-1 doi.org/10.1515/chem-2015-0087; January 13, 2015. [6] G.Thomas, “Modern electron microscopy for materials characterization,” J. Electron Microsc. Tech., vol. 3, no. 1, pp. 95–108, doi: 10.1002/jemt.1060030109;1986. [7] Gwenael Gouadec and Philippe Colomban “Raman Spectroscopy of Nanomaterials: How Spectra Relate to Disorder, Particle Size and Mechanical Properties’’, Progress in Crystal Growth & Characterization of Materials” vol 53, issue 1, pp.1-56 ;doi.org/10.1016/j.pcrysgrow.2007.01.001;2007. [8] Gybina A.A., Prohaska J.R.,“Copper deficiency results in AMP-activated protein kinase activation and acetyl CoA carboxylase phosphorylation in rat cerebellum”, Natural library of medicine, Brain Res. 2008;1204:69–76;doi: 10.1016/j.brainres.2008.01.087;2008. [9] J.-M. Herrera-Ramirez, Ph. Colomban and A. Bunsell, “Micro-Raman Study of the Fatigue Fracture and Tensile Behaviour of Polyamide (PA 66) Fibres,” J. Raman Spectrosc.vol-35, issue 12; pp.1063-1072;doi.org/10.1016/j.engfracmech.2006.04.033 dec-2004. [10] Jadhav SV, Bringas E, Yadav GD, Rathod VK, Ortiz I, Marathe KV (2015),“ Arsenic and fluoride contaminated groundwaters: a review of current technologies for contaminants removal”. J Environ Manag vol-162; pp.306–325;doi.org/10.1016/j.jenvman.2015.07.020 oct 2015. [11] Jie Ma, Mengya Sun, Yulan Zeng, Zhenhua Liu, Manmam Zhang, Yao Xiao and Shuping Zhang “Acetylacetone functionalized magnetic carbon microspheres for the highly-efficient adsorption of heavy metal ions from aqueous solutions”.RSC Advance;vol 9;pp.3337-3344;doi.org/10.1039/C8RA09830A;2019. [12] Kailash R. Jagdeo,Bholanath T. Mukherjee, Suyash S. Agnihotri, Vikaskumar P. Gupta,“Bimetal decorated carbon nano material synthesized from waste cotton (plant based precursor) for enhanced hydrogen uptake capacity,”International Journal of Creative Research Thoughts -IJCRT(IJCRT.ORG) vol-9; issue;2021 [13] Kampa M., Castanas E., “Human health effects of air pollution”.Environ.Pollut.;vol-151;issue-2;pp362–367. doi: 10.1016/j.envpol.2007.06.012. 2008 [14] Kunduru KR, Nazarkovsky M, Farah S, Pawar RP, Basu A, Domb AJ “ Nanotechnology for water purification: applications of nanotechnology methods in wastewater treatment”, Water purification; pp 33–74;doi.org/10.1016/B978-0-12-804300-4.00002-2;2017 [15] L Anah and N Astrini,“Influence of pH on Cr(VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent”. IOP Conf. Series: Earth and Environmental Science 60 (2017) 012010 doi:10.1088/1755-1315/60/1/012010;2017 [16] M Samuel Collin, Senthil Kumar Venkatramam, Naveensubramaniam Vijaykumar and etal, “Bioaccumulation of lead (Pb) and its effects on human: A review”, Journal of Hazardous Materials Advances. Volume 7,100094;doi.org/10.1016/j.hazadv.2022.100094;2022. [17] Nady A. Fathy , Altaf H. Basta , Vivian F. Lotfy “ 4 - Novel trends for synthesis of carbon nanostructures from agricultural wastes”.Carbon Nanomaterials for Agri-Food and Environmental Applications :pp59-74;doi.org/10.1016/B978-0-12-819786-8.00004-9:2020. [18] P. M. Shukla and S. R. Shukla , “Biosorption of Cu (II), Pb (II), Ni (II), and Fe (II) on alkali treated coir fibers”, Sep. Sci. and Technol;vol 48;issue-3; pp.421- 428;doi.org/10.1080/01496395.2012.691933;2013. [19] Palin, D., Jr; Rufato, K. B.; Linde, G. A.; Colauto, N. B.; Caetano, J.; Alberton, O.; Jesus, D. A.; Dragunski, D. C.“Evaluation of Pb (II) biosorption utilizing sugarcane bagasse colonized by Basidiomycetes”. Environ. Monit. Assess. , 188, 279– 293, doi: 10.1007/s10661-016-5257-8,2016. [20] Qu X, Alvarez PJJ, Li Q ,“ Applications of nanotechnology in water and wastewater treatment”,Water Research;vol 47;issue 12;pp3931–3946;doi.org/10.1016/j.watres.2012.09.058;2013. [21] Reglero M.M., Taggart M.A., Lidia M.G., Rafael M.,“ Heavy metal exposure in large game from a lead mining area: Effects on oxidative stress and fatty acid composition in liver.” Environ. Pollut.;vol-157;issue-4;pp.1388–1395. doi: 10.1016/j.envpol.2008.11.036;2009. [22] Sinicropi M.S., Caruso A., Capasso A., Palladino C., Panno A., Saturnino C.,“ Heavy metals: Toxicity and carcinogenicity”. Pharmacologyonline;vol-2:pp329–333;2013. [23] T.Theivasanthi and M. Alagar,“X-Ray diffraction Studies of Copper Nanopowder”,Scholars Research Library,Archives of physics Research,vol 1;issue-2,pp.112-117;doi.org/10.48550/arXiv.1003.6068;2017;2010. [24] Vendruscolo F, da Rocha Ferreira GL, Antoniosi Filho NR.,“ Biosorption of hexavalent chromium by microorganisms”. Int Biodeterior Biodegrad.vol 119;pp.87–95;doi.org/10.1016/j.ibiod.2016.10.008;2017. [25] Weng C-H, Sharma YC, Chu S-H,“Adsorption of Cr(VI) from aqueous solutions by spent activated clay”. J Hazard Materials vol.155issue.(1–2):pp.65–75;doi.org/10.1016/j.jhazmat.2007.11.029;2008 [26] Z. Yong, Z. Zhu, Z. Wang, J. Hu, and Q. Pan, “One-dimensional carbon nanotube–FexCy nanocrystal composite,” Nanotechnology, vol. 18, no. 10, Article ID 105602;doi.org/10.1088/0957-4484/18/10/105602;2007. [27] Z.-Z. Zhu, Z. Wang, and H.-L. Li, “Functional multi-walled carbon nanotube/polyaniline composite films as supports of platinum for formic acid electrooxidation,” Applied Surface Science, vol. 254; issue 10;pp. 2934–2940;doi.org/10.1016/j.apsusc.2007.10.033;2008. [28] Zhang Y, Wu B, Xu H, Liu H, Wang M, He Y, Pan B (2016),“ Nanomaterials-enabled water and wastewater treatment.” NanoImpact vol.3-4;pp-23-39;doi.org/10.1016/j.impact.2016.09.004;2016.
Copyright
Copyright © 2023 Bholanath T. Mukherjee, Arati D. Nandapure, Pooja O. Jayswal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET56385
Publish Date : 2023-10-30
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

